- Show Menu
- Contact Us
- FAQs
- Reader Service
- Survey Data
- Survey Winners
- Testimonials
- Upcoming Events
- Webinars
- White Papers
New & Noteworthy
April 2010
Subcutaneous Fluid Administration
Baxter International, Inc
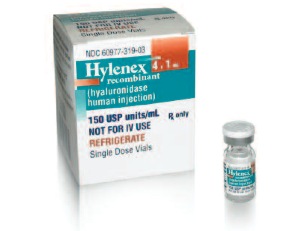
Baxter introduces HYLENEX, an FDA-approved human recombinant hyaluronidase for subcutaneous fluid administration in pediatrics patients. Unlike IV administration, HYLENEX is administered under the skin without the need to secure a vein for treatment initiation. HYLENEX temporarily makes the tissue beneath the skin more permeable, allowing fluids to be more readily absorbed into the blood vessels. HYLENEX has been shown to be safe and effective in infants and young children with mild to moderate dehydration.
Recent Popular Articles
About Us
Pharmacy Purchasing & Products Ridgewood Medical Media, LLC
Quick Links
Subscribe to Our Email Newsletter!
© 2005 - 2025 PP&P Magazine - Pharmacy Purchasing & Products.
All rights reserved.