- Show Menu
- Contact Us
- FAQs
- Reader Service
- Survey Data
- Survey Winners
- Testimonials
- Upcoming Events
- Webinars
- White Papers
New & Noteworthy
January 2022
Arsenic Trioxide Injection
Amneal Pharmaceuticals
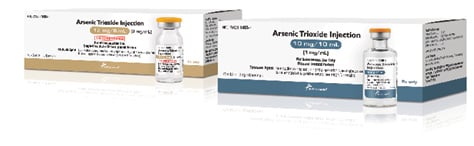
Amneal Pharmaceuticals releases FDA-approved arsenic trioxide injection, the generic equivalent for Trisenox. Arsenic trioxide is indicated for induction of remission and consolidation in patients with acute promyelocytic leukemia (APL) who are refractory to, or have relapsed from, retinoid and anthracycline chemotherapy, and whose APL is characterized by the presence of the t(15;17) chromosomal translocation or PML/RAR-alpha gene expression. The injection is packaged in ten 10 mg/10 mL (1 mg/mL) and 12 mg/6 mL (2 mg/mL) single-dose vials.
Recent Popular Articles
About Us
Pharmacy Purchasing & Products Ridgewood Medical Media, LLC
Quick Links
Subscribe to Our Email Newsletter!
© 2005 - 2025 PP&P Magazine - Pharmacy Purchasing & Products.
All rights reserved.